Given :
2N2O5(g) → 4NO2(g) + O2(g)
Rate of disappearance of N2O5 = 0.015 Ms-1
Rate of formation of NO2 = ?
Rate of formation of O2 = ?
Rate of reaction = ?
Rate of disappearance of N2O5
= \(\frac{-d[N_2O_5]}{dt}\)
= 0.015 Ms-1
Since 4 moles of NO2 are formed from 2 moles of N2O5 Rate of formation of NO2
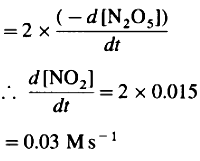
Rate of formation of O2,
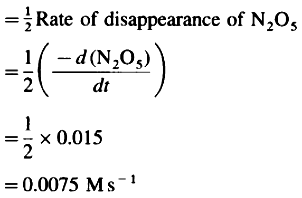

∴ Rate of formation of NO2 = 0.03 Ms-1
Rate of formation of O2 = 0.0075 Ms-1
Rate of reaction = 0.0075 Ms-1.