
When Carbon dioxide is passed through freshly prepared lime water, it forms water and white precipitate of Calcium carbonate because of which lime water turns milky.
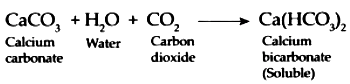
When Carbon dioxide is continuously passed through milky lime water, it forms Calcium bicarbonate which is soluble in water and therefore, water once again turns colourless.