
2-bromobutane is a 2° alkylhalide whereas 1-bromobutane is a 1° alkyl halide. The approaching of nucleophile is more hindered in 2-bromobutane than in 1-bromobutane. Therefore, 1-bromobutane reacts more rapidly than 2-bromobutane by an SN2 mechanism.
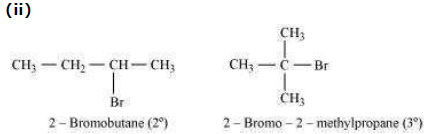
2-Bromobutane is 2° alkylhalide whereas 2-bromo-2-methylpropane is 3° alkyl halide.
Therefore, greater numbers of substituents are present in 3° alkyl halide than in 2° alkyl
halide to hinder the approaching nucleophile. Hence, 2-bromobutane reacts more rapidly
than 2-bromo-2-methylpropane by an SN2 mechanism.

Both the alkyl halides are primary. However, the substituent −CH3 is at a greater distance
to the carbon atom linked to Br in 1-bromo-3-methylbutane than in 1-bromo- 2methylbutane. Therefore, the approaching nucleophile is less hindered in case of the former than in case of the latter. Hence, the former reacts faster than the latter by SN2 mechanism.