Mass of a negatively charged muon
According to Bohr’s model,


Hence, the value of the first Bohr radius of a muonic hydrogen atom is 2.56 × 10−13 m.
We have,
Ee= − 13.6 eV
Take the ratio of these energies as:
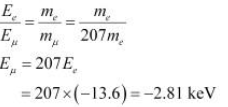
Hence, the ground state energy of a muonic hydrogen atom is −2.81 keV.