For an experiment, a scientist fills different gases in four flasks as shown below:
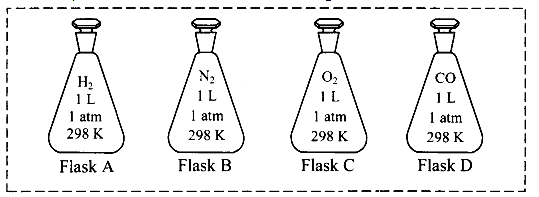
i. What is the ratio of the number of molecules of the gases in flask A to flask B?
ii. Calculate the pressure exerted by nitrogen gas in flask B if the temperature is doubled.
iii. If the scientist transfers the gas in flask D to another flask of 2.5 L at 1 atm pressure, what will be the temperature of the gas in the new flask?