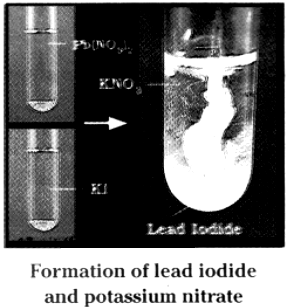
It forms yellow precipitate of lead iodide.
1. Take a pinch of lead nitrate and dissolve in 5.0 ml of distilled water in a test tube.
2. Take a pinch of potassium iodide in another test tube and dissolve in distilled water.
3. Mix lead nitrate solution with potassium iodide solution.
4. We observe that a yellow coloured substance which is insoluble in water, is formed.
5. This insoluble substance is known as precipitate.
6. The precipitate is Lead Iodide.
7. Equation : Fb(NO3)2(aq) + 2 KI(aq) → Pbl2(s) + 2KNO3(aq)
8. In the above reaction, lead ion and potassium ion exchange their places each other.
9. Lead ion combines with iodide ion and forms Pbl2 as precipitate and KNO3 remains in the solution.
10. Such reaction is double displacement reaction.