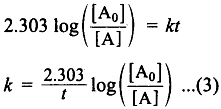A reaction whose fate depends on the reactant concentration raised to the first power is called a first order reaction. Let us consider the following Cl2 first-order reaction,
A → product
Rate law can be expressed as Rate = k [A]1
Where, k is the first order rate constant.

Integrate the above equation between the limits of time t = 0 and time equal to t, while the concentration varies from the initial concentration [A0] to [A] at the later time.
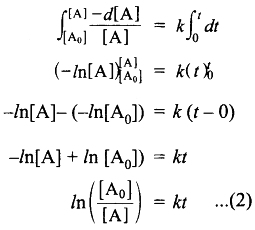
This equation is in a natural logarithm. To convert it into the usual logarithm with base 10, we have to multiply the term by 2.303.