Liebig’s method for calculating carbon and hydrogen.
In Leibig’s method, a known mass of an organic substance is fully oxidised using cupric oxide. CO2 and H2O are formed when the C and H are oxidised. By passing CO2 and H2O generated through a hyd. CaCl2 tube and a KOH solution tube, the mass of CO2 and H2O created may be calculated. The percent of C and H is computed using these masses of CO2 and H2O.

Procedure:
In Liebig’s method, a known quantity of the organic substance is heated vigorously in the presence of pure oxygen, with the carbon present being oxidised to carbon dioxide and the hydrogen being oxidised to water. The chemical reaction of combustion is as follows.
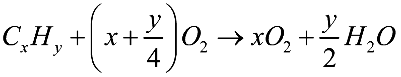
After passing through a U-shaped tube holding anhydrous calcium chloride, the combustion products are sent to the caustic potash container. Caustic potash interacts with carbon dioxide gas when anhydrous calcium chloride absorbs the water content.
The carbon dioxide and water that are produced are weighed and collected.
The mass of carbon dioxide and water generated is used to compute the proportion of carbon and hydrogen in the chemical.
The equipment for Liebig’s test is depicted in the diagram below :

The conversion of carbon and hydrogen in an organic substance to carbon dioxide and water is used to estimate their amounts. As a result, Liebig’s test is appropriate for estimating the amount of carbon and hydrogen in organic molecules.
Note: If nitrogen is present in the organic component, it is oxidised to nitrogen oxides, which are also absorbed in caustic soda bottles under combustion circumstances. In such circumstances, reduced copper gauze is put near the tube’s exit end, converting nitrogen oxides to nitrogen gas.