3. (3) Constant
Given,
P-T diagram
Density of gas will be constant as from graph pressure is directly proportional to the temperature
For ideal gas PV = nRT
From this relation V is constant
\(Density = \frac {mass}{volume}\)
As mass and volume both are constant so it's density will also constant.
4. (2) Increases continuously
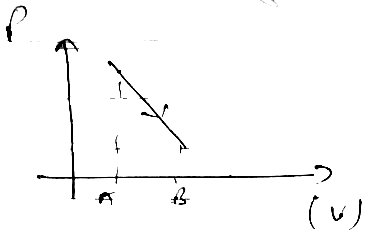
PV = nRT
P/V = const = K
P = KV
⇒ (KV)V = nRT
⇒ V2 = CT
VA < VB
TA < TB
Increases