Based on reactions with AgNO3 and (en), and optical activity, isomers can be identified.
First form : There is no reaction with AgNO3, hence no Cl– ions outside coordination sphere. Also there is no reaction with bidentate (en), hence these ligands are trans to each other. Optical inactivity is also due to trans structure. Thus, it may have structure :

trans-chloronitrobis (ethylenediamine) cobalt (III) nitrite.
Secon form : In this Cl– is outside coordination sphere since it reacts with AgNO3. As in the first form NO2 – ligands are trans to each other being optically inactive. This is represented as,

trans-bis (ethylenediamine) dinitrocobalt (III) chloride
Third form : In this case also, Cl– is outside coordination sphere. Also it shows reaction with (en) hence monodentate ligands are cis to each other. Being optically active, mirror image should not superimpose. Thus, it can have structure :
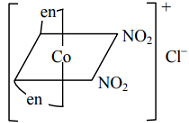
cis-bis (ethylenediamine) dinitrocobalt (III) chloride