The relative strength of weak acids and bases are generally determined by their dissociation constants Ka and Kb respectively. For weak acid, i.e. CH3COOH
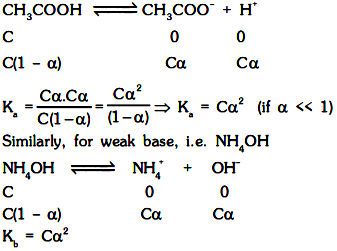
Ka and Kb are just the equilibrium constants and hence depends only on temperature. Greater the value of dissociation constant of the acid (Ka), more is the strength of the acid and similarly greater the value of dissociation constant of the base, more is the strength of the base. For two acids of equimolar concentrations.

The modern method is to convert Ka as a power of 10 and express acid strength by power of 10 with sign changed and call this new unit pKa. Thus, if Ka for acid is equal to 10–4, pKa = 4. So higher pKa value means lower acid strength,
that is, pKa = – log Ka
Also, pKb = – log Kb