Take a beaker of 250 ml and place two nails fixed with the help of cork.
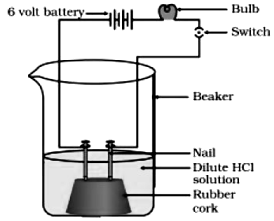
• Connect the nails to the two terminals of a 6 volt battery as shown in figure.
• Now add some water containing ethanol and put the switch ON.
• Repeat the experiment with glucose solution.
Observation : K The bulb will not glow and the needle of ammeter will not show deflection because glucose and ethanol do not conduct electricity.
Conclusion: The experiment shows glucose and ethanol do not ionise in aqueous solution, that is, they do not give H+ ions, therefore cannot conduct electricity. Thus, glucose and ethanol are not categorised as acids.