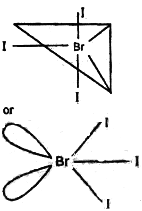
The central atom Br has 7 Valence Electron. Three of these will form covalent bond with three F-atom leaving behind for electrons. Thus there are three bond pairs and two lone pairs. According to VSEPR- Theory, these bonds will occupy the corners of a trigonal bipyramid two lone pairs will occupy equatorial position to minimise lone pair- lone pair and B.P.- L.P. repulsion, which are greater than B.P.-B.P. repulsion. In addition, the axial F-atom will be bent towards the equatorial F in order to minimise the L.P.-L.P. repulsion, Therefore shape would be slightly bent T.