(CH3)2 CHN = NCH(CH3)2(g) → N2(g) + C6H14(g)
Suppose pi is the initial pressure at t = 0
After time t say x mole of azoisopropane decomposes and the pressure is p.
(CH3)2 CHN = NCH(CH3)2 → N2 + C6H4
Intial pressure (at t = 0) Pi 0 0
pressure at time = t Pi – x x x
The rate constant at 360 s,

Pi = 35.0 mm
At time t, p = pi – x + x + x = Pi + x
x = p – Pi = 54 – 35 = 19.0 mm
Now p at time 360s = 35 – 19.0 = 16 mm
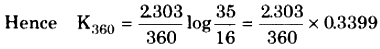
= 2.17 x 10-3 s-1
Similarly x = p - pi = 63 – 35 = 28 mm
p at time 720 sec = 35 – 28 = 7 mm
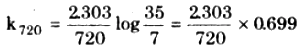
= 2.235 x 10-3 s-1