H2O undergoes extensive intermolecular hydrogen bonding due to greater electro negativity of O than S. So H2O exist as an associated molecules. A larger amount of energy is required to break these hydrogen bonds. Therefore H2O is a liquid at room temperature.
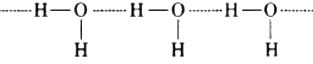
In contrast, H2S does not undergoes hydrogen bonding as the electro negativity difference between H and S is not appreceable. It exist as discrete molecule which are held together by weak vander Waal’s force of attraction. To break these forces of attraction, only a small amount of energy is required. Therefore. H2S is a gas at room temperature.