General chemical reactions of glucose can be grouped under two categories:
(a) Reactions due to alcoholic groups:
There are four secondary and one primary alcoholic groups present in a molecule of glucose. Some of the reactions of alcoholic groups are as follows:
(i) Acetylation:

(ii) Methylation:
Glucose reacts with methyl alcohol in the presence of dry HCl gas forming methyl glucoside which is an ether.

(b) Reactions due to aldehydic (-CHO) group:
(i) Reduction:
When reduced with Na-Hg and water, the -CHO group in glucose is reduced to a primary alcoholic group to give sorbitol.

When reduction is carried with HI and red P, the product is n-hexane. This suggests that all six carbon atoms in glucose are linked to form a straight chain.

(ii) Oxidation:
With mild oxidising agent like Br2 water, glucose is oxidised to gluconic acid which is a carboxylic acid. This shows that the carbonyl group present in glucose is an aldehydic group.

In case the oxidation is carried out with conc. HNO3 the primary alcoholic group is also oxidised to carboxyl group to form a dicarboxylic acid which is saccharic acid.

(iii) Reaction with hydrogen cyanide:
Glucose forms a cyanohydrin derivative with hydrogen cyanide.
(iv) Reaction with hydroxylamine:
Glucose reacts with hydroxylamine to form an oxime.

(v) Action with Tollen’s Reagent:
When Tollen’s reagent is heated with glucose in a clean test tube, a shining mirror gets deposited at the bottom of the test tube.
AgNO3 + NH4OH → AgOH + NH4NO3
AgOH + 2NH4OH → Ag(NH3)2OH + 2H2O

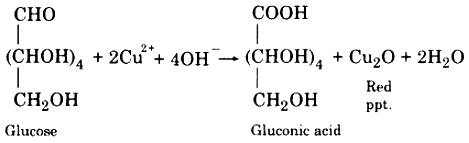
(vi) Action with Fehling’s Solution:
When heated with glucose, Fehling solution gives a red precipitate.
(vii) Reaction with Phenyl Hydrazine:
Glucose contains in it an aldehydic group and is expected to react with phenylhydrazine to form phenylhydrazone. However when the excess of phenylhydrazine is used the product formed is an osazone i.e., glucosamine.
