(i) Lead (II) chloride reacts with Cl2 to give PbCl4 because lead exists in +2 and +4 oxidation states.
PbCl2 + Cl2 ➝ PbCl4
(ii) Lead tetrachloride (PbCl4) is highly unstable towards heat because the stability of +4 oxidation state decreases on moving down the group in the periodic table due to inert pair effect. So, +2 oxidation state of Pb is more stable. It decomposes on heating to give lead dichloride and chlorine gas.
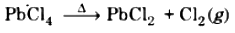
(iii) Concentrated nitric acid reacts with aluminium to form a very thin layer of aluminium oxide which acts as a protective layer and protects from further reaction of nitric acid with aluminium. Due to this protective layer aluminium cannot react further with cone. HNO3. Hence, concentrated nitric acid can be transported in aluminium container.
(iv) Graphite has layered structure in which different layers are held together by weak Vander Waals forces and so each layer can slip over one another and hence graphite is used as a lubricant.