Different types of chemical reactions are:
(a) Combination reactions: The reaction in which two or more than two substances combine together to form a single compound.
e.g., 2Mg + O2 → 2MgO
(b) Decomposition reaction: The reaction in which a compound decomposes to form two or more substances is called decomposition reaction.
e.g., CaCO3  CaO + CO2
CaO + CO2
(c) Displacement reaction: The reaction in which more reactive metal displaces the less reactive metal is called displacement reaction.
e.g., Fe + CuSO4 → FeSO4 + CO2
(d) Double displacement reaction: The reaction in which two different atoms or groups of (substances) atoms exchange for each other is called double displacement reaction.
e.g., NaO2SOO4(aq) + BaClO2(aq) → BaSOO4(s) + 2NaCl(aq)
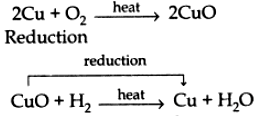
(e) Oxidation-reduction reaction: The reaction in which oxygen is added/ hydrogen is removed is called oxidation reaction.
The reaction in which hydrogen is added/oxygen is removed is called reduction reaction. e.g., Oxidation,