Isomers are the compound which has the same molecular formula but different structure formula. Ieomers of Butane :
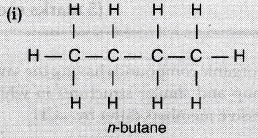

We cannot have isomers of the first three members of the alkane series because of the following laws of isomers :
(i) The parent chain should have the most number of carbon atoms.
(ii) The branching cannot be done from the first on the last atom-carbon atom of the structure