Variation of electron negativity in a period: The electro negativity increases across a period from left to right. Since the atomic radius decreases in a period, the attraction between the valence electron and the nucleus increases. Hence the tendency to attract shared pair of electrons increases. Therefore, electro negativity increases in a period.

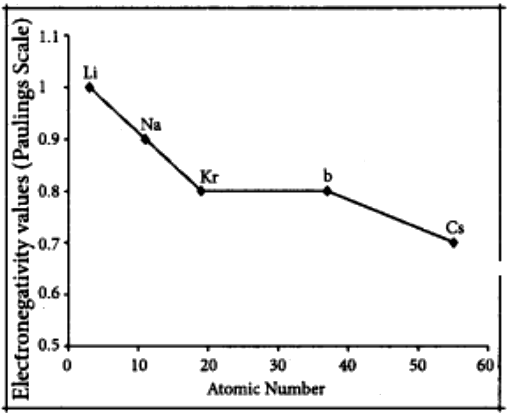
Variation of electro negativity in a group: The electro negativity decreases down a group. As we move down a group, the atomic radius increases and the nuclear attractive force on the valence electron decreases. Hence electro negativity decreases in a group.