The electrolytic refining of copper is done using the apparatus shown in the figure below.
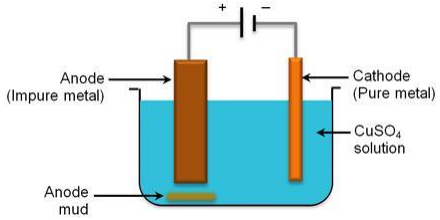
This is a standard electrolysis setup, where the impure copper (the sample to be refined) is placed as anode and a thin strip of pure copper is placed as cathode. The electrolyte used is copper sulphate solution. When current is passed through the electrolyte, pure copper content from anode dissolves into the electrolyte, and the same amount of copper is deposited on the cathode. One can see that the thickness of the cathode increases.
The soluble impurities present in the anode goes into the solution when the current flows through the setup. The insoluble impurities present in the anode settle down at the bottom of the anode and is called anode mud. Thus, one can refine copper electrolytically.